Better
Treatments,
More Tomorrows
Better
Treatments,
More Tomorrows
We are
Advancing
A late-stage pipeline of product candidates for the treatment of hard-to-treat tumors and viruses.

Innovative
Therapies
Lead program, Annamycin, planning to commence the “MIRACLE” pivotal Phase 3 clinical trial with the potential to transform the standard of care for 2nd line acute myeloid leukemia (AML).
Innovative
Therapies
Lead program, Annamycin, planning to commence the “MIRACLE” pivotal Phase 3 clinical trial with the potential to transform the standard of care for 2nd line acute myeloid leukemia (AML).

AML Clinical Day Featuring Clinicians Martin S. Tallman, MD and Michael Andreeff, MD, PhD
May 7, 2024
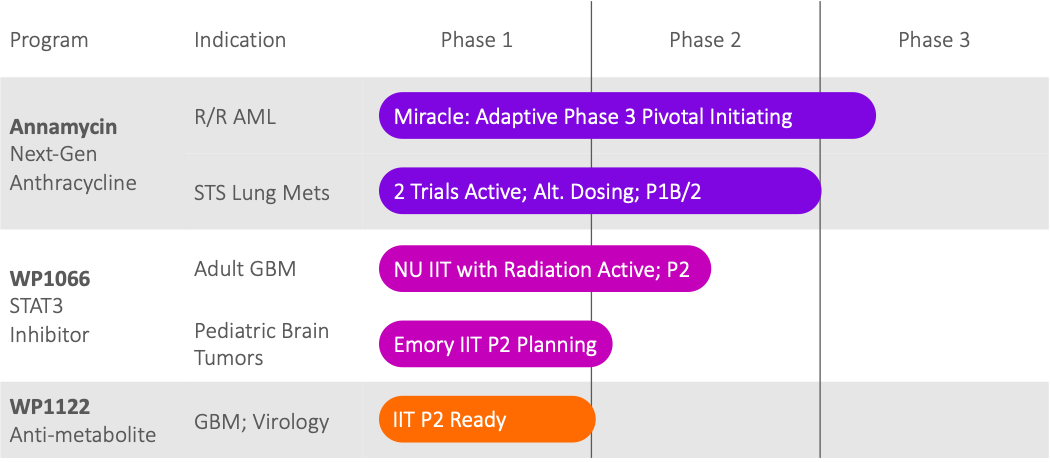
Priority Development Pipeline
AML Patient Journey and the Annamycin Opportunity
Latest Releases
Stock Information
NASDAQ: MBRX
NASDAQ: MBRX
Corporate
Presentation
Corporate
Presentation
