Annamycin is a next-generation anthracycline being evaluated in the MIRACLE (Moleculin R/R AML AnnAraC Clinical Evaluation) Trial, a pivotal, adaptive design Phase 3 trial evaluating Annamycin in combination with cytarabine, together referred to as AnnAraC, for the treatment of relapsed or refractory acute myeloid leukemia (AML). If successful, Annamycin has the potential to transform the standard of care for 2nd line AML. Annamycin is also in development for the treatment of soft tissue sarcoma (STS).
We believe anthracyclines are among the most important treatments for AML and advanced STS. However, cardiotoxicity issues limit their effectiveness with life-time maximum dosing set by the US FDA.
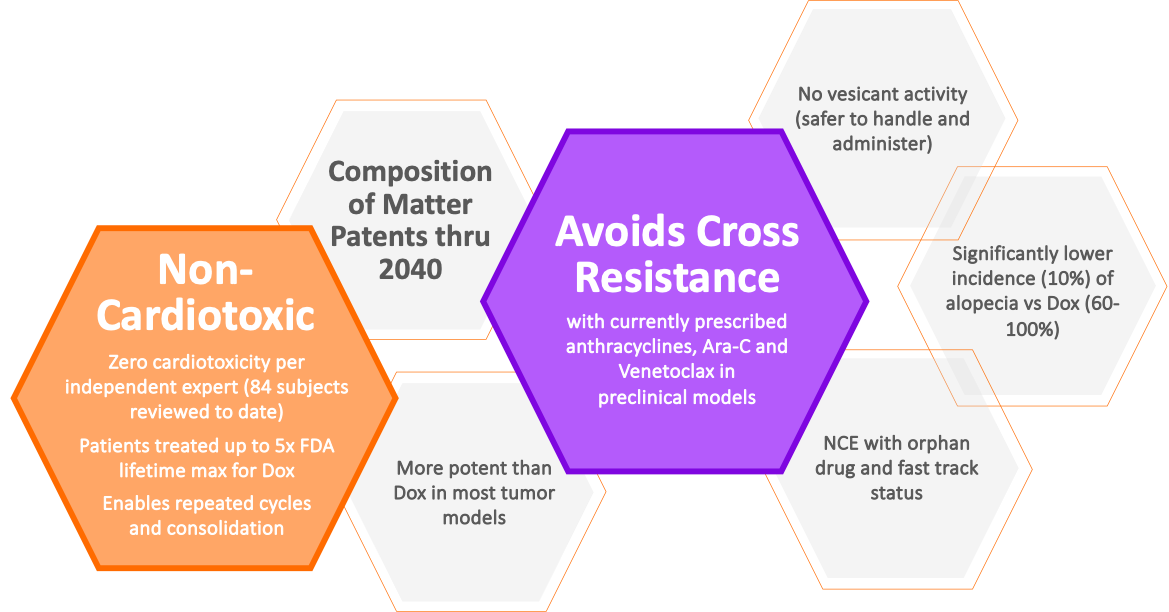
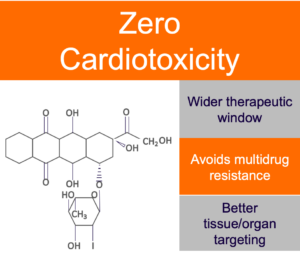
Annamycin has demonstrated in multiple clinical trials to have zero cardiotoxicity, allowing us to exceed the currently set maximum lifetime dosing for an anthracycline and potentially realizing the full therapeutic potential of anthracyclines to treat serious, hard-to-treat cancers.
In addition to showing zero cardiotoxicity in patients treated in Moleculin trials to date, Annamycin is demonstrating to have superior efficacy in 2nd line AML compared to currently available treatments.
Key Highlights:
- ZPotential to be safer and more effective than current prescribed anthracyclines
- ZNon-cardiotoxic and avoids cross resistance with doxorubicin, cytarabine (Ara-C) and Venetoclax
- ZDelivers more than double the Complete Response (CR) rate of any approved treatment for relapse/refractory acute myeloid leukemia
- ZUS FDA Orphan Drug Designation for STS Lung Mets and AML U.S. FDA Fast Track Status for STS Lung Mets
Active Clinical Studies:
Acute Myeloid Leukemia
MIRACLE: Pivotal, Adaptive Phase 3 Clinical Study
Soft Tissue Sarcoma
MB-107: Phase 1B/2 Study
Key Annamycin Attributes:
Notes: 1) Current Cardiology Review, Anthracycline Cardiotoxicity: Prevalence, Pathogenesis and Treatment, Maria Volkova and Raymond Russel III. Referenced from Cancer. 2003 Jun 1;97(11):2869-79. “Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials”. Swain SM, Whaley FS, Ewer MS., PMID: 12767102; 2) Preliminary clinical studies from Moleculin; data subject to change; 3) Refer to Form 10K for FYE 2023 for discussion on latest subject with an increase in troponins and our Expert’s opinion. The MIRACLE study is subject to appropriate future filings with and potential additional feedback from the FDA and their foreign equivalents.
Annamycin Has Demonstrated Substantially Greater Cardiac Safety Compared to Approved Anthracyclines
Current Anthracyclines
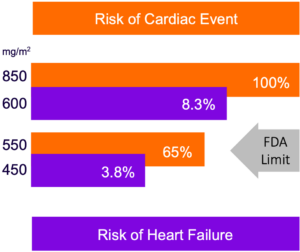
Annamycin
Notes: 1) Current Cardiology Review, Anthracycline Cardiotoxicity: Prevalence, Pathogenesis and Treatment, Maria Volkova and Raymond Russel III. Referenced from Cancer. 2003 Jun 1;97(11):2869-79. “Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials”. Swain SM, Whaley FS, Ewer MS., PMID: 12767102; 2) Preliminary clinical studies from Moleculin; data subject to change; 3) Refer to Form 10K for FYE 2023 for discussion on latest subject with an increase in troponins and our Expert’s opinion.
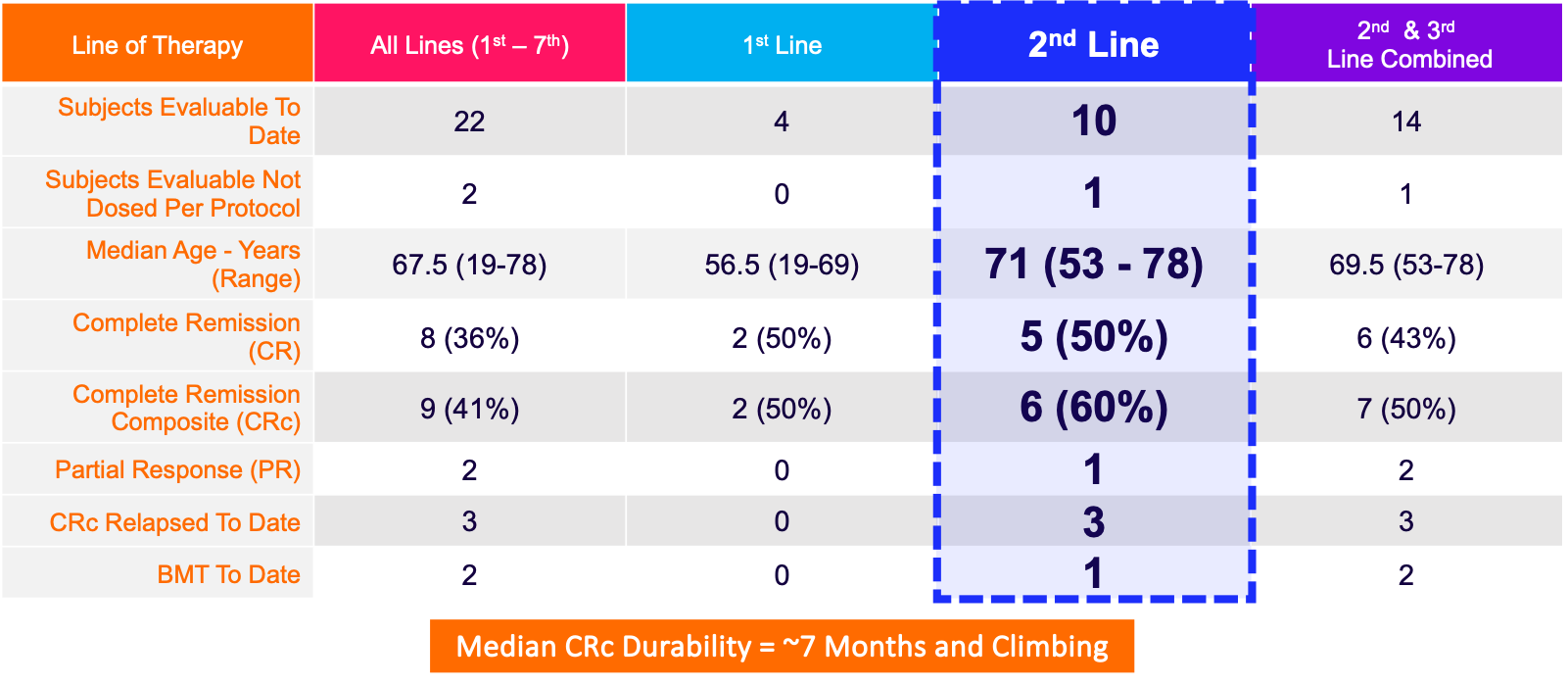
Demonstrated Positive Results from Phase 1B/2 Study in AML (MB-106)
Notes: 1) Data from MB-106 are for intent to treat subjects who had efficacy determined (n=22); 2) Data from MB-106 are preliminary and subject to change; 3) Durability is developing; and 3) Relapses include 1 death due to pneumonia (unrelated to drug).
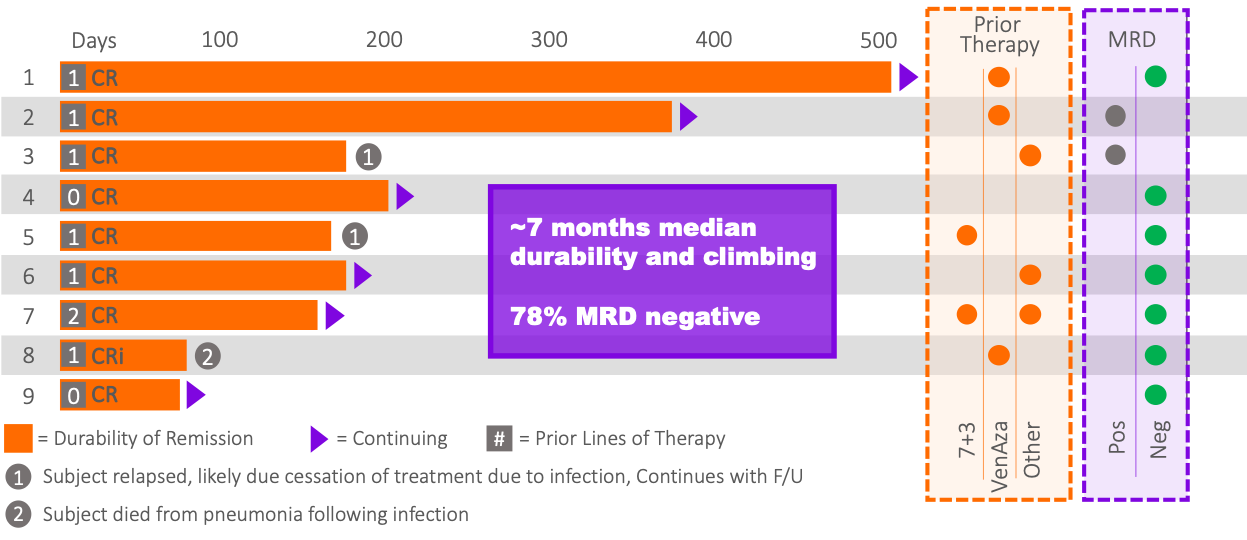
Durability, MRD, Prior Therapies (MB-106)
Notes: 1) MB106 data is preliminary and subject to change until a Clinical Study Report is published. 2) MRD testing is not standard between sites.
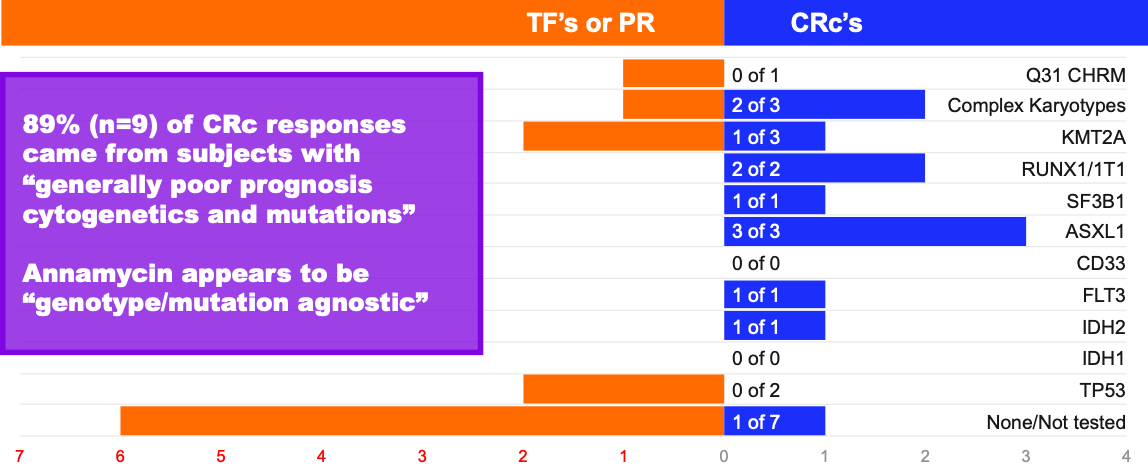
Response by Genotype and Mutation (MB-106)
Note – n=20; Some subjects had multiple mutations or abnormalities, hence totals of treatment failures (TF), partial remissions (PR) or composite complete remissions (CRc) do not equal totals for each response category – TF’s/PR’s, or CRc’s; Data are anecdotal only and not intended to indicate statistical significance. Not all mutations/subjects were tested.