We believe anthracyclines are among the most important treatments for AML and advanced STS. However, cardiotoxicity issues limit their effectiveness with life-time maximum dosing set by the US FDA.
Annamycin has demonstrated in multiple clinical trials a lack of cardiotoxicity1, allowing us to exceed the currently set maximum lifetime dosing for an anthracycline and potentially realizing the full therapeutic potential of anthracyclines to treat serious, hard-to-treat cancers.
In addition to demonstrating a lack of cardiotoxicity1 in patients treated in Moleculin trials to date, Annamycin is demonstrating to have superior efficacy in 2nd line AML compared to currently available treatments.
Key Highlights:
✔ Potential to be safer and more effective than current prescribed anthracyclines
✔ Non-cardiotoxic1 and avoids cross resistance with doxorubicin, cytarabine (Ara-C) and Venetoclax
✔ Delivers more than double the Complete Response (CR) rate of any approved treatment for relapse/refractory acute myeloid leukemia
✔ US FDA Orphan Drug Designation for STS Lung Mets and AML U.S. FDA Fast Track Status for STS Lung Mets
Active Clinical Studies:
Acute Myeloid Leukemia
MIRACLE: Pivotal, Adaptive Phase 3 Clinical Study
Soft Tissue Sarcoma
MB-107: Phase 1B/2 Study
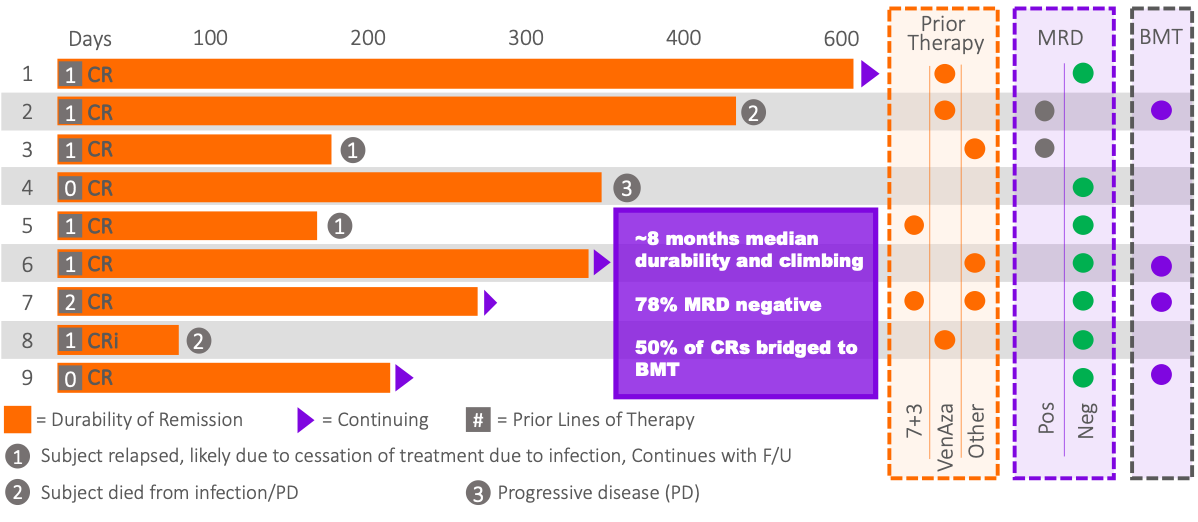
AnnAraC Performance in R/R AML
1 – Median; 2L subjects (n=10) 2 – Median; 1-3L responders (n=9)
It is widely recognized that the majority of 2L AML patients are underserved by available approved therapies and that better options are needed for this unmet need.
We believe the performance of AnnAraC is significantly better than any therapy ever approved for use in 2L AML.
Annamycin Attributes:
Annamycin Has Demonstrated Substantially Greater Cardiac Safety Compared to Approved Anthracyclines
Current Anthracyclines
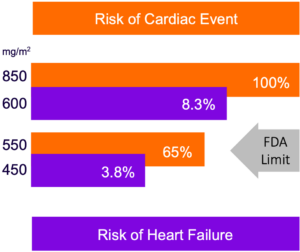
Annamycin
Source Current Cardiology Review, Anthracycline Cardiotoxicity: Prevalence, Pathogenesis and Treatment, Maria Volkova and Raymond Russel III. Referenced from Cancer. 2003 Jun 1;97(11):2869-79. “Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials”. Swain SM, Whaley FS, Ewer MS., PMID: 12767102; Preliminary clinical studies from Moleculin.
MB-106 (Annamycin + Ara-C (AnnAraC); n=22)
Notes: 1) Data from MB-106 are for intent to treat subjects who had efficacy determined (n=22); 2) Data from MB-106 are preliminary and subject to change; 3) Durability is developing; and 3) Relapses include 1 death due to pneumonia (unrelated to drug).
Durability, MRD, Prior Therapies
1) MB106 data are preliminary and subject to change until a Clinical Study Report is published. 2) MRD testing is not standard between sites
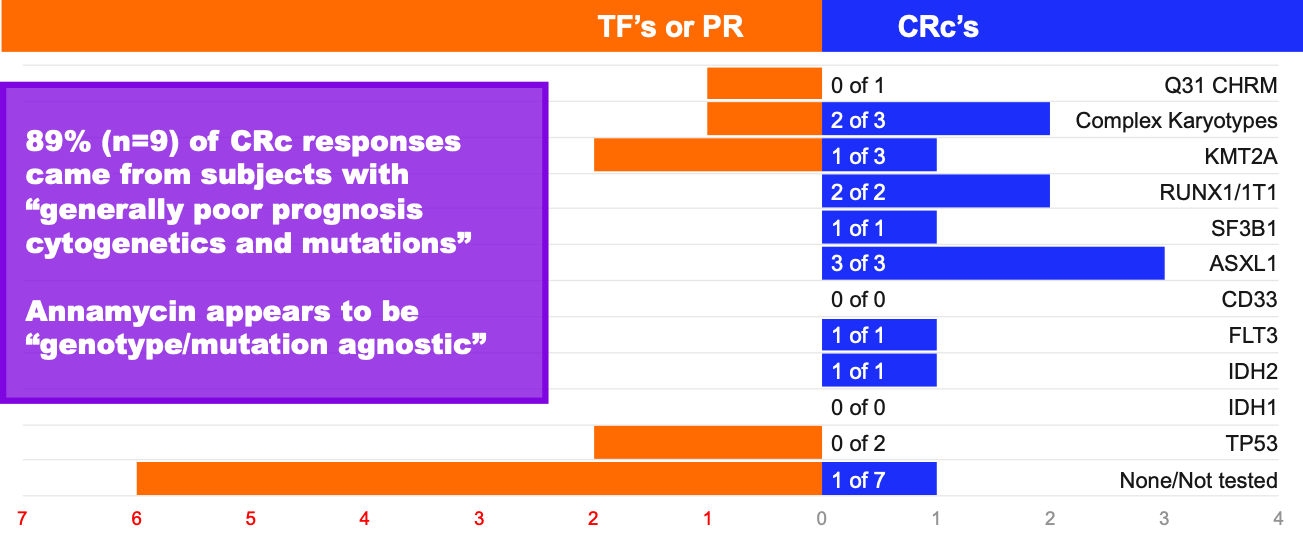
MB-106 Response by Genotype and Mutation
Note – n=20; Some subjects had multiple mutations or abnormalities, hence totals of treatment failures (TF), partial remissions (PR) or composite complete remissions (CRc) do not equal totals for each response category – TF’s/PR’s, or CRc’s; Data are anecdotal only and not intended to indicate statistical significance. Not all mutations/subjects were tested.
1: Moleculin Biotech, Inc. (2024). Form 10-K for the fiscal year ended December 31, 2024. https://moleculin.com/sec-filings/. For updates on Annamycin and its lack of cardiotoxicity, see subsequent disclosures in Forms 8-K, 10-Q, and 10-K.