Completed Study: MB-106
Phase 1B/2 Study of Annamycin in Combination with Cytarabine (Ara-C) in AML
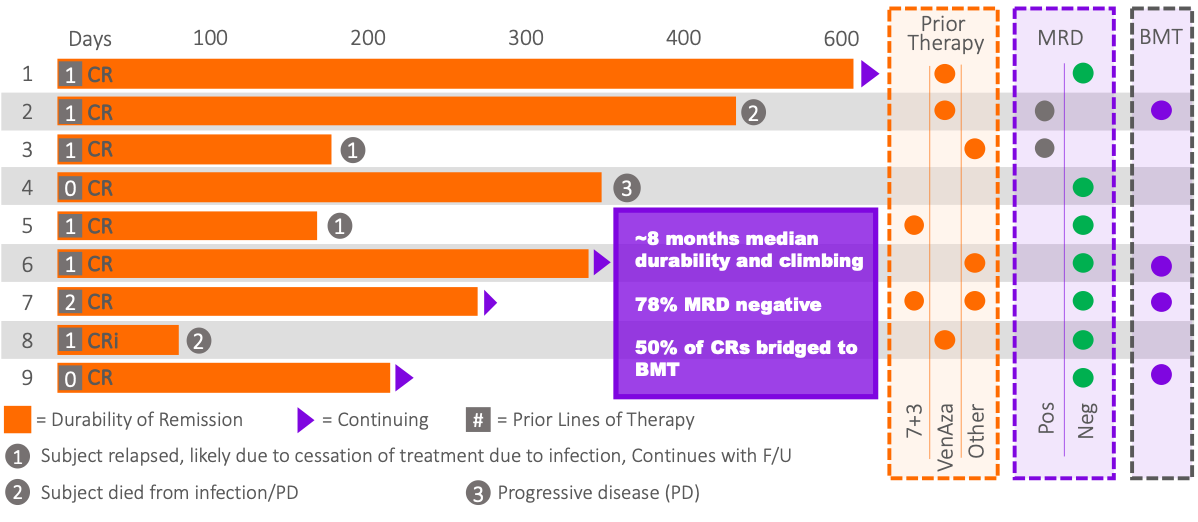
As of June 2024, a total of 22 subjects have been enrolled (the Intent-to-Treat population, ITT) and evaluated, with 9 subjects (41%) achieving a composite complete remission (CRc or CR/CRi), including 8 (36%) subjects with complete remission (CR) and one subject with complete remission with an incomplete recovery (CRi), following treatment with AnnAraC. Of the 10 ITT subjects for whom AnnAraC was administered in the 2nd line setting, 5 achieved a CR (50%) and 6 achieved a CRc (60%). Of all 22 subjects in the ITT evaluable population that were 2nd or 3rd line treatment, 6 achieved a CR (43%) and 7 achieved a CRc (50%). The median durability of response (mDOR) for the 9 subjects who achieved a CRc is approximately 7 months and climbing. Additionally, the median overall survival for all 22 ITT subjects evaluable is approximately 7 months and increasing. 78% of the CRc responders were also MRD negative. MRD stands for Measurable Residual Disease and being MRD negative is considered by many clinicians to be an important positive indicator of the likelihood of long-term patient success.
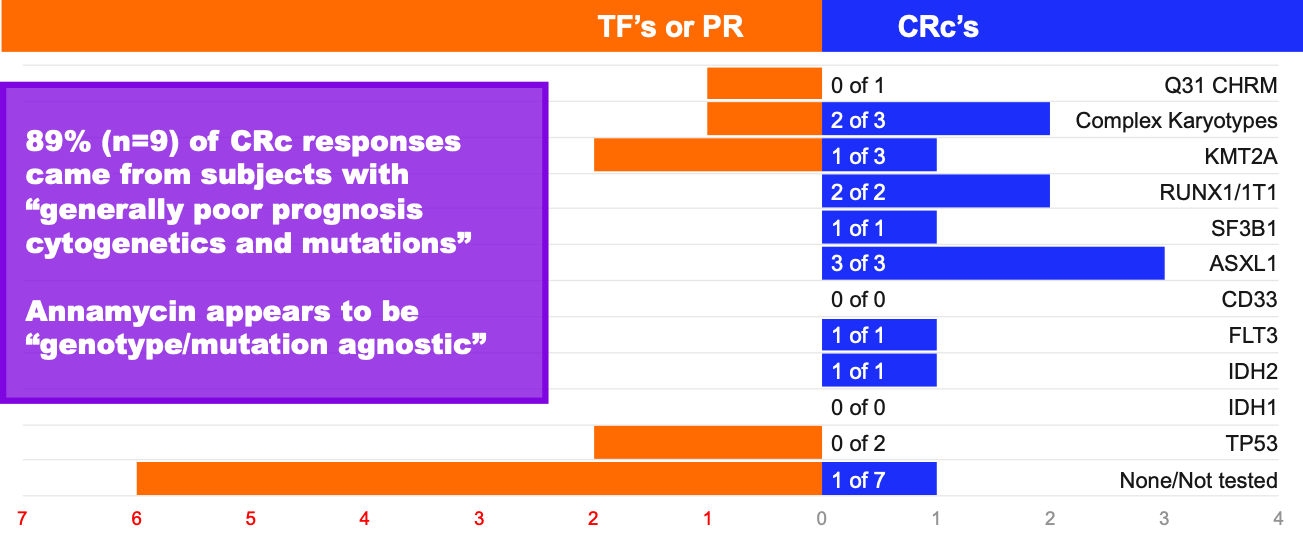
Additionally, 89% of the subjects with a CRc (n=10) had cytogenetics and mutations that are generally associated with a poor prognosis. These include FLT3, IDH2, ASXL1, KMT2A and others. While not statistically relevant, the Company believes such data are informative to clinicians.
MB-106 Response by Genotype and Mutation
Note – n=20; Some subjects had multiple mutations or abnormalities, hence totals of treatment failures (TF), partial remissions (PR) or composite complete remissions (CRc) do not equal totals for each response category – TF’s/PR’s, or CRc’s; Data are anecdotal only and not intended to indicate statistical significance. Not all mutations/subjects were tested.
The median age of subjects in MB-106 was 69 years. Not including the two most recent subjects, a total of 17 subjects had relapsed/refractory AML and 3 subjects were first line treatment. Two subjects discontinued early due to allergic reactions. All subjects who completed treatment had undergone post-therapy bone marrow assessment (Day 15 or later). No clinically significant signs of cardiotoxicity were noted during or after treatment in any of the subjects enrolled. The combination was well tolerated with myelosuppression and infections being the main adverse events (AEs). All data from MB-106 is preliminary and subject to change.
For complete study details, please visit clinicaltrialsregister.eu: EudraCT 2020-005493-10 or clinicaltrials.gov: NCT05319587