Ongoing Study:
MB-107: Phase 1B/2 Study of Annamycin in STS Lung Mets in the United States
Multi-Center, Open-Label, Single-Arm Study of Liposomal Annamycin for the Treatment of Subjects with Soft-Tissue Sarcomas (STS) with Pulmonary Metastases
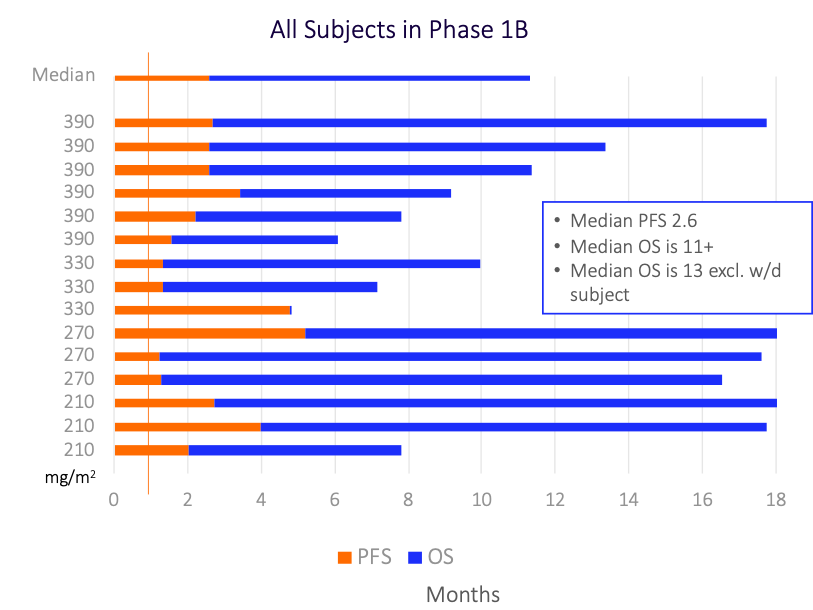
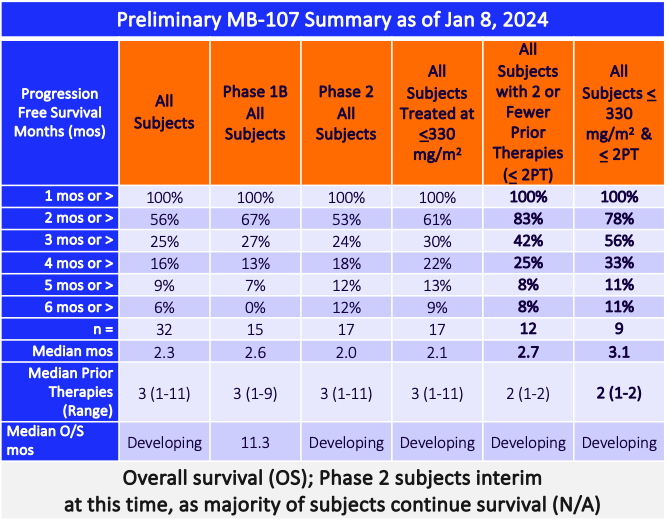
Preliminary Phase 1B Data Demonstrated No Cardiotoxicity1
and Demonstrated Clinical Activity2
Study Highlights:
Enrolling: ~25 Subjects in Phase 2 portion
Treatment Period: Single dose every 21 days
RP2D: 360mg/m2 (protocol provides for a reduction to 330 mg/m2 after the first 3 patients treated if warranted based on tolerability)
Phase 1B Concluded: RP2D determined to be 360mg/m2
Phase 2 Recruitment Open: Expansion at 360 mg/m2 with option to decrease to 330 mg/m2
For complete study details, please view the study listing on ClinicalTrials.gov Identifier: NCT04887298
1: Moleculin Biotech, Inc. (2024). Form 10-K for the fiscal year ended December 31, 2024. https://moleculin.com/sec-filings/. For updates on Annamycin and its lack of cardiotoxicity, see subsequent disclosures in Forms 8-K, 10-Q, and 10-K.
2: Data are preliminary. Defined as stable disease or better. As of August 12, 2022, preliminary data demonstrated 12 of 14 patients measured had SD after two cycles of Annamycin.