MIRACLE: Pivotal, Adaptive Phase 3 Trial in Acute Myeloid Leukemia
The MIRACLE study, subject to appropriate future filings with and potential additional feedback from the FDA and their foreign equivalents, is expected to initially utilize an adaptive design whereby the first 75 to 90 subjects will be randomized in Part A of the trial to receive high dose cytarabine (HiDAC) combined with either placebo, 190 mg/m2 of Annamycin, or 230 mg/m2 of Annamycin, such doses were specifically recommended by the FDA in the Company’s end of Phase 1B/2 meeting. The amended protocol will allow for the unblinding of preliminary primary efficacy data (CR) and safety/tolerability of the three arms at 45 subjects. This early unblinding will yield 30 subjects with Annamycin (190mg/m2 and 230/m2) and HiDAC and 15 subjects with just HiDAC. The Company expects to reach 45 subjects in the second half of 2025, in addition to the planned unblinding expected in 2026 of the next 30-45 subjects.
For Part B of the trial, approximately 244 additional subjects will be randomized to receive either HiDAC plus placebo or HiDAC plus the optimum dose of Annamycin. The selection of the optimum dose will be based on the overall balance of safety, pharmacokinetics and efficacy, consistent with the FDA’s new Project Optimus initiative. This increase from 240 to 244 subjects represents the statistical “cost” of the additional unblinding.
The amended protocol is currently being reviewed by the Institutional Review Board (IRB). Once approved, the amended protocol will be filed with the amendment for the Company’s Initial New Drug (IND) application in the US with the FDA.
Annamycin currently has Fast Track Status and Orphan Drug Designation from the FDA for the treatment of relapsed or refractory acute myeloid leukemia, in addition to Orphan Drug Designation for the treatment of soft tissue sarcoma. Furthermore, Annamycin has Orphan Drug Designation for the treatment of relapsed or refractory acute myeloid leukemia from the European Medicines Agency (EMA).
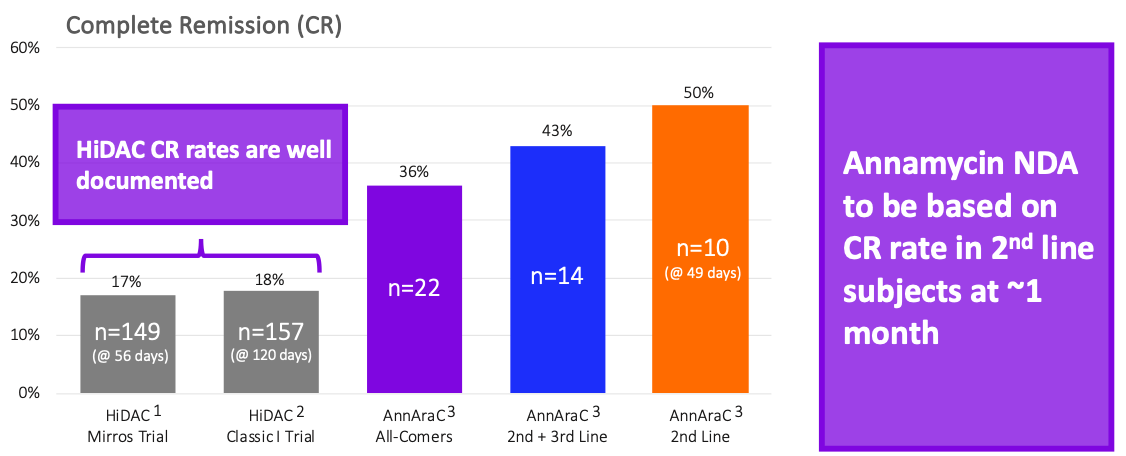
The Bar for Approval is Low
1 – Mirros Trial, 81% 2nd line patients; 17% CR, within 56 days, Konopleva et al, Blood Advances, 26 July 2022, Volume 6, Number 14; 2 – Classic I Trial, 18% CR rate within 120 days, Faderl et al, J Clin Oncol, July 2012, Volume 30, Number 20; 3 – MB-106 trial, 50% CR rate for 2nd line patients (n=10, within 49 days), 43% CR rate for 2nd + 3rd line patients (n=14), and 36% CR rate for all-comers (1st through 7th line, n=22)
The MIRACLE trial is subject to appropriate future filings with and potential additional feedback from the FDA and their foreign equivalents. Annamycin currently has Fast Track Status and Orphan Drug Designation from the U.S. Food and Drug Administration for the treatment of relapsed or refractory acute myeloid leukemia, in addition to Orphan Drug Designation for the treatment of soft tissue sarcoma. Furthermore, Annamycin has Orphan Drug Designation for the treatment of relapsed or refractory acute myeloid leukemia from the European Medicines Agency (EMA). For more information about the MB-106 Phase 1B/2 trial, visit clinicaltrialsregister.eu and reference EudraCT 2020-005493-10 or clinicaltrials.gov and reference NCT05319587.