Based on an encouraging discussion in the End of Phase 1B/2 Meeting with FDA the Company plans to:
Proceed with a pivotal, adaptive Phase 3 clinical trial (the “MIRACLE” trial) designed for possible accelerated approval of Annamycin in combination with cytarabine for the treatment of relapsed or refractory AML;
Run such future studies globally and in the US above the lifetime maximum allowable anthracycline dose; and
Provide the FDA with additional data supporting the selection of the optimal dosing level via the adaptive design in the MIRACLE trial
HOUSTON, Aug. 1, 2024 — Moleculin Biotech, Inc., (Nasdaq: MBRX) (“Moleculin” or the “Company”), a clinical stage pharmaceutical company with a broad portfolio of drug candidates targeting hard-to-treat tumors and viruses, today announced the positive discussion in and outcome of its End of Phase 1B/2 (EOP1B/2) meeting with the US Food and Drug Administration (FDA) supporting the advancement of Annamycin in combination with Cytarabine (also known as “Ara-C” and for which the combination of Annamycin and Ara-C is referred to as “AnnAraC”) to a Phase 3 pivotal trial for the treatment of AML patients who are refractory to or relapsed after induction therapy (R/R AML). This Phase 3 “MIRACLE” trial (derived from Moleculin R/R AML AnnAraC Clinical Evaluation) will be a global trial, including sites in the US.
“We thank the FDA’s Divisions of Hematologic Malignancies I and Cardiology and Nephrology, as well as related divisions, for a very constructive EOP1B/2 meeting and for their valuable feedback. Armed with this, we are now able to finalize plans for a pivotal approval pathway in AML,” commented Walter Klemp, Chairman and Chief Executive Officer of Moleculin. “Importantly, consistent with the FDA’s recommendations, the adaptive Phase 3 trial will rely solely on CR (complete remission) at day 30 as the primary endpoint versus placebo, a standard we are confident Annamycin will meet and that provides an opportunity for accelerated approval.”
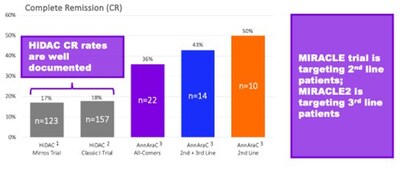
Mr. Klemp continued: “We now also have additional confidence that our planned pivotal trial should be able to generate data supportive of a true value inflection point for shareholders in a timely manner. We plan to utilize a double-blind, placebo-controlled design, where the control arm is high dose cytarabine (HiDAC) plus placebo. There is considerable historical data on the use of HiDAC. You can see in this graphic that, compared to this historical data, AnnAraC has already demonstrated more than double the CR rate. The MIRACLE trial will initially focus on 2nd line treatment for R/R AML subjects and then follow-up with treatment for 3rd line R/R AML.”
“This approach should also allow us to use this trial for approval in Europe. Based on our discussions with the FDA, we intend to amend our current investigational new drug application or IND to allow dosing above the lifetime maximum allowable dose (LTMAD) for currently prescribed anthracyclines in this trial in the US.”
The Company obtained valuable input from the FDA and having resolved a number of key issues, believes that it has significantly de-risked the pathway to approval. The MIRACLE study, subject to appropriate future filings with and potential additional feedback from the FDA and their foreign equivalents, is expected to initially utilize an adaptive design whereby the first 75 patients will be randomized to receive HiDAC combined with either placebo, 190 mg/m2 of Annamycin, or 230 mg/m2 of Annamycin. At that point, the trial will be unblinded to select the Optimum Dose for Annamycin. For the second half of the trial, approximately 120 additional patients will be randomized to receive either HiDAC plus placebo or HiDAC plus the Optimum Dose of Annamycin. The selection of the Optimum Dose will be based not only on the absence of dose limiting toxicities but also on the overall balance of safety, pharmacokinetics and efficacy, consistent with the FDA’s new Project Optimus initiative.
Mr. Klemp concluded: “The FDA also wants to see the durability of response (DoR) and overall survival (OS) as secondary endpoints, as well as data for patients beyond 2nd line, which is why our plan includes a follow-on MIRACLE2 trial in 3rd line patients starting once the optimum dose is established in the MIRACLE trial. From a Company perspective, we believe this approach is the best of all worlds. We are not only making the leap into being a Phase 3 company, but our planned approval is also based on a primary endpoint comparing to a control that we are optimistic we can beat with the ability to report unblinded progress after just 75 patients. We are truly excited to launch the MIRACLE trial.”
Moleculin Planned Significant Milestones
The Company has established plans for the following milestones:
- 2H 2024 – Begin contracting with MIRACLE trial sites
- Q1 2025 – First subject treated in MIRACLE trial
- Mid 2026 – Interim data (n=75) unblinded and Optimum Dose set for MIRACLE trial
- 2026 – Begin enrollment of 3rd line subjects in MIRACLE2
- 2027 – Enrollment ends in 2nd line subjects
- 2028 – Final Data for 2nd line subjects in MIRACLE
- 2H 2028 – Begin submission of a new drug application (NDA) the treatment of R/R AML for accelerated approval on primary endpoint of CR from MIRACLE
Annamycin currently has Fast Track Status and Orphan Drug Designation from the US Food and Drug Administration for the treatment of relapsed or refractory acute myeloid leukemia, in addition to Orphan Drug Designation for the treatment of soft tissue sarcoma. Furthermore, Annamycin has Orphan Drug Designation for the treatment of relapsed or refractory acute myeloid leukemia from the European Medicines Agency (EMA). For more information about the ongoing MB-106 Phase 1B/2 trial, visit clinicaltrialsregister.eu and reference EudraCT 2020-005493-10 or clinicaltrials.gov and reference NCT05319587.
About Moleculin Biotech, Inc.
Moleculin Biotech, Inc. is a clinical stage pharmaceutical company with a growing pipeline, including Phase 2 clinical programs, for hard-to-treat tumors and viruses. The Company’s lead program, Annamycin is a next-generation anthracycline designed to avoid multidrug resistance mechanisms and to eliminate the cardiotoxicity common with currently prescribed anthracyclines. Annamycin is currently in development for the treatment of acute myeloid leukemia (AML) and soft tissue sarcoma (STS) lung metastases. All interim and preliminary data related to its active clinical trials are subject to change until a clinical study report is published.
Additionally, the Company is developing WP1066, an Immune/Transcription Modulator capable of inhibiting p-STAT3 and other oncogenic transcription factors while also stimulating a natural immune response, targeting brain tumors, pancreatic and other cancers. Moleculin is also engaged in the development of a portfolio of antimetabolites, including WP1122 for the potential treatment of viruses, as well as certain cancer indications.
For more information about the Company, please visit www.moleculin.com and connect on X, LinkedIn and Facebook.
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Although Moleculin believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Moleculin has attempted to identify forward-looking statements by terminology including ‘believes,’ ‘estimates,’ ‘anticipates,’ ‘expects,’ ‘plans,’ ‘projects,’ ‘intends,’ ‘potential,’ ‘may,’ ‘could,’ ‘might,’ ‘will,’ ‘should,’ ‘approximately’ or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under Item 1A. “Risk Factors” in our most recently filed Form 10-K filed with the Securities and Exchange Commission (SEC) and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. We undertake no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
Investor Contact:
JTC Team, LLC
Jenene Thomas
(833) 475-8247
MBRX@jtcir.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/moleculin-announces-plans-for-miracle-phase-3-pivotal-trial-302211451.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/moleculin-announces-plans-for-miracle-phase-3-pivotal-trial-302211451.html
SOURCE Moleculin Biotech, Inc.